Electrochemistry is a branch of chemistry that provides insight into the interactions between electrical and chemical effects. At its core, electrochemistry explores the transformation of chemical substances through electrical processes, paving the way for groundbreaking applications across diverse fields. Let’s explore electrochemistry and its fundamental principles, captivating discoveries, and transformative potential.
Understanding Electrochemistry:
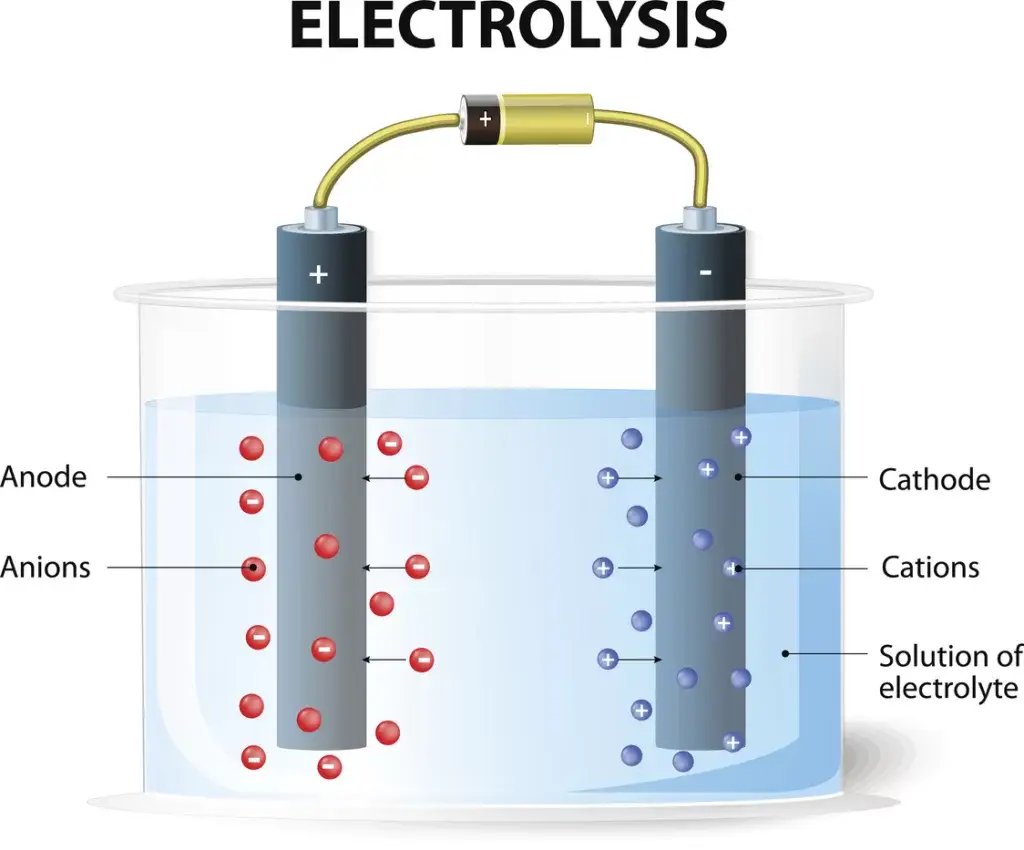
Electrochemistry revolves around reduction and oxidation reactions, or “redox” reactions, where chemical species transfer electrons. These reactions occur within electrochemical cells, with the anode facilitating oxidation (losing electrons) and the cathode facilitating reduction (gaining electrons). When a voltage is applied to the electrochemical cell, electrons flow between these electrodes, generating an electric current and powering various electrochemical processes.
Key Concepts and Phenomena:
Electrochemistry encompasses essential concepts and phenomena, upon which scientific inquiry and technological innovation rely.
Electrochemical Cell: An electrochemical system consisting of two electrodes and an electrolyte (Fig. 1). A voltage is applied across the electrodes to drive a chemical reaction.
Electrode: An electronic conductor. Electrons carry charge through the electrode.
Anode: A positively charged electrode, commonly a solid metal. The anode loses electrons.
Cathode: A negatively charged electrode, also commonly a solid metal. The cathode gains electrons.
Electrolyte: An ionic conductor that is frequently a liquid containing ionic species, such as sodium (Na+) and chloride (Cl-). Ions carry charge through the electrolyte.
Electrolysis: Uses electrical energy to drive non-spontaneous chemical reactions, decomposing substances into ions or elements.
Electroplating or Electrodeposition: Deposits a thin layer of metal, or coating, onto a substrate through electrolysis, extensively used in automotive and electronics industries for example.
Batteries and Fuel Cells: Store and convert chemical energy directly into electrical energy, powering devices from portable electronics to electric vehicles and energy storage systems.
Corrosion: Degrades metals by oxidizing the surface, a reaction that occurs naturally when materials interact with their environment (e.g., iron oxidizes in water to form iron oxide, which is rust). This can have detrimental impacts on industries and infrastructure.
Applications and Impact:
Electrochemistry permeates everyday life and drives technological advancements.
Green Energy: Powers renewable energy technologies like lithium-ion batteries, solar cells, and hydrogen production, contributing to sustainable energy solutions.
Healthcare: Facilitates medical devices, biosensors, and drug delivery systems, advancing diagnostics and treatment.
Environmental Remediation: Treats wastewater, controls pollution, and remediates contaminated sites for environmental sustainability.
Materials Science: Controls material properties, leading to innovations in coatings, nanomaterials, and semiconductor fabrication.
Future Frontiers:
Without question, electrochemistry will play an important role in our future. Below are just a few areas where continued innovation can improve our quality of life.
Electrochemical Synthesis: Sustainably synthesizes chemicals and materials for various applications (e.g., disinfectants and rubber materials).
Energy Storage: Advances storage technologies for enhanced performance and scalability in energy storage systems (e.g., solar cells, lithium-ion batteries, and fuel cells).
Electrochemical Sensing: Develops high-sensitivity sensors for detecting and monitoring analytes in diverse fields (e.g., glucose biosensors and carbon monoxide detectors).
Electrochemical Conversion: Explores pathways for converting greenhouse gasses into fuels and chemicals for climate change mitigation (e.g., conversion of carbon dioxide (CO2) to useful chemicals)
In conclusion, electrochemistry bridges chemistry, physics, material science, and engineering, offering unlimited opportunities for research and innovation. From powering devices to advancing environmental sustainability, electrochemistry continues to shape a brighter future.
