ElectraMet™ Challenge
A steel factory roll forms, washes, and coats steel coils. Hexavalent chromium, Cr(VI), is added to alloy steel to increase hardenability and corrosion resistance. Cr(VI) compounds may also be used as pigments in dyes, paints, inks, and plastics. However, Cr(VI) is tightly regulated because it is highly soluble in water and known to cause cancer.
Background
An industrial facility’s chromium wastewater was being transported off site for third-party disposal at a substantial cost. The customer was seeking a more efficient process for on-site chromium wastewater treatment.
ElectraMet Cr(VI) Treatment Process
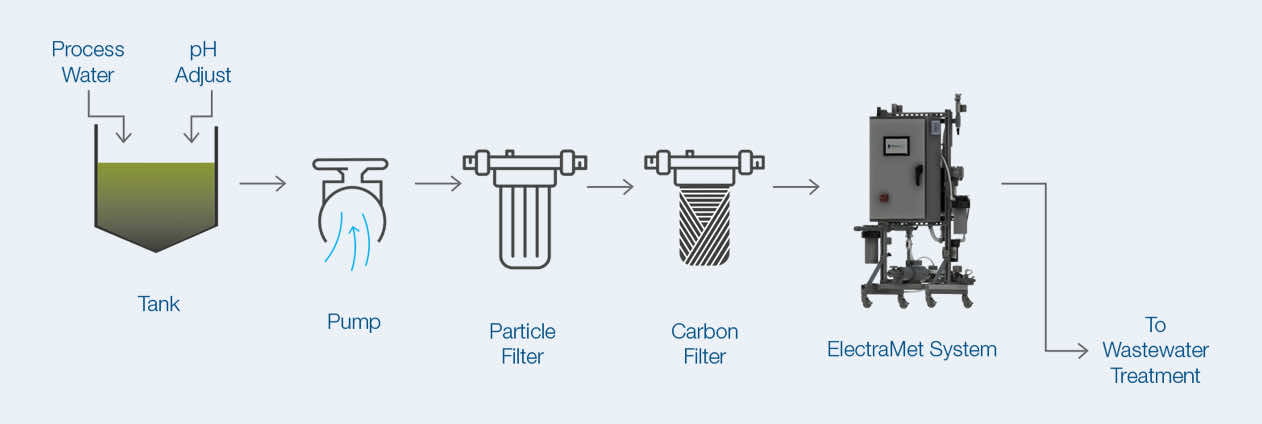
Wastewater coming from chrome electroplating or coating processes with dissolved hexavalent chromium, Cr(VI), up to 2.5 g/L was treated by ElectraMet to meet the discharge requirement. ElectraMet uses electricity and applied voltage to reduce Cr(VI) to Cr(III), which is more reliable than sodium bisulfite or other chemical reduction processes and reduces the total volume of chromium-containing waste as the wastewater passes through the ElectraMet cartridge. ElectraMet replaces the need for chemicals (e.g. sodium bisulfite) for the same purpose. Removing sodium bisulfite and other chemicals improves the efficiency of downstream wastewater treatment processes and reduces the overall volume of sludge for disposal.
Results
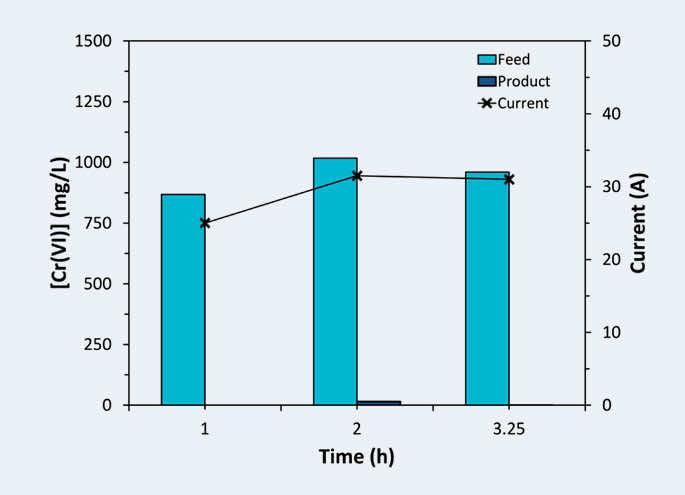
Process effectiveness is measured at the job site in several ways. Influent Cr(VI) containing water is a yellow color, while the treated Cr(III) containing water is green Figure 2). ElectraMet shows voltage and current response as Cr(VI) is reduced to Cr(III). If water entering the ElectraMet system contains no chromium, the system does not draw electrical current. Current response is therefore a direct measure of the chromium reduced as wastewater passes through the ElectraMet system (Figure 3). After ElectraMet reduces Cr(VI) to Cr(III), the customer’s subsequent treatment steps include lime addition until the water is pH > 7 to precipitate Cr(III) as an oxide or hydroxide, followed by clarification and filter press to produce a cake.
With the ElectraMet system, the customer significantly saves time and money by eliminating the need for third party chrome wastewater disposal. ElectraMet’s automated system reduces the risk and burden of labor intensive treatment to ensure discharge compliance. Additionally, ElectraLink™ and ElectraMet engineers provide the customer with monitoring analytics and ongoing support for the lifetime of the system.