The pursuit of sustainability presents a challenge: “How can we make the old, new?” ElectraMet is rising to the occasion through optimized waste valorization (recovering assets from waste streams)
The Basics of Electrowinning
Useful materials exist in unusable forms all around us, for example, industrial process wastewater often contains trace amounts of valuable metals in the form of dissolved ions, such as copper, silver, and many others. In this aqueous form, these elements go to waste like the water they are dissolved in. However, these dissolved ions can be removed to create solid, usable metal through a process called “electrowinning.”
Invented in the 19th century, electrowinning is the process of applying an electric across two electrodes in the presence of water. The process uses electric current to split water and donate electrons from the hydrogen to the metal ions, thereby “winning” electrons for use in converting the metal ion to a metal solid.
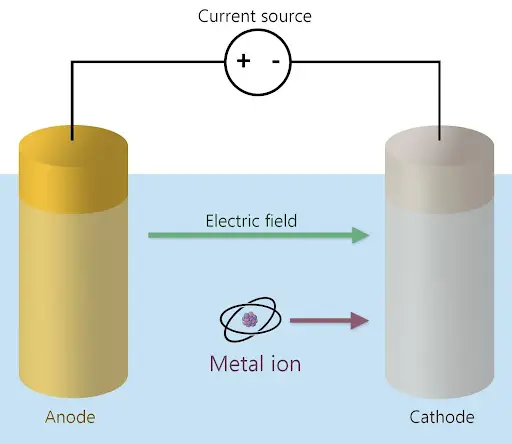
Electrowinning takes place in an “electrolytic cell. To create an electrolytic cell, two electrodes, an anode and a cathode, are placed in the solution. The anode is positively charged (+), while the cathode is negatively charged (-). A voltage is created across these electrodes through the application of a current to the electrodes. This in turn creates an electric field pointing in the direction of the cathode.
The voltage ionizes the atoms, which means they lose one or more of their electrons, turning them into ions. For metals, these ions are positively charged, so they move in the direction of the electric field, closer to the negatively charged cathode, as seen in Figure 1. The ions then undergo a reduction reaction in which they gain electrons by electrodeposition or electroplating onto the cathode. The result is a layer of pure metal upon the cathode’s surface which can be collected and repurposed.
Shortcomings of Traditional Electrowinning versus ElectraMet’s Technology
Traditional electrowinning has significant limitations which lead to process failure and safety issues. Issues and Impacts are listed below along with how ElectraMet represents a revolution in the use of electrochemistry to solve metal ion removal and recovery challenges.
Potential Issue #1: The materials and/or equipment used to form the electrolytic cell can become damaged. Over time, the electrodes can corrode, much like metal rusting when exposed to water. This failure leads to removal efficiencies which are typically balanced with increased current, which increases the risk of bridging.
Solution to Issue #1: ElectraMet R&D and patent process identified the right mix of materials, flow, and controls to ensure robust operation for an extended duration. Different materials are used for different pH ranges and acid types to address material compatibility.
Potential Issue #2: The electrode can increase in operating temperature and eventually fail due to bridging. Bridging is the direct connection between the anode and cathode when too much material accumulates on the cathode. These start as dendrites and eventually build a bridge toward the electron generating source. When this occurs, the current no longer moves through the solution; instead, the current is directed through the bridge from the cathode to the anode, much like a wire in a circuit. This overloads the anode with energy, causing it to short-circuit and fail through burning or melting.
Solution to Issue #2: ElectraMet addresses this common failure mode by using advanced analytics and material separators within its cells. The analytics provide clairvoyance on electrolytic cell operation enabling regeneration sequences ahead of issues. Separators ensure proper spacing of the anode from the cathode to eliminate the possibility of bridging.
Potential Issue #3: Accumulation of impurities in the electroplated metal occurs due to lack of voltage potential control. When multiple metals are dissolved in the solution, they each deposit on the cathode’s surface, creating a mixed metal or alloy.
Solution to Issue #3: ElectraMet’s technology avoids this altogether by taking advantage of the decomposition voltage potential of the targeted metals. This enables the customer to remove targeted metals in a predictable fashion to generate pure metal or pure metal salts.
ElectraMet’s technology has removed copper, silver, manganese and other metals to < 30 ppb levels in industrial applications to meet discharge compliance and protect water reuse membranes semiconductor and other industries.
Learn More about ElectraMet
ElectraMet is disrupting the wastewater treatment industry by developing innovative, robust, and sustainable solutions to age-old problems. ElectraMet’s system is efficient, cost-effective, and versatile.
If you’d like to learn more about electrowinning and the revolutionary technology offered by ElectraMet, please visit our Knowledge Hub or contact us today!
